Can blue light burn up body fat?
Yes, there are light sensitive pigments residing in adipose cells
President’s Day ‘Blue Light Special’ is all about what good the UV light spectra can do for you.
Adipose Cells soak it up to burn it up
Full sunlight beaming on you as you sunbathe can penetrate full skin thickness and affect the fat cells residing in your dermis. These cells contain nonimaging extraretinal opsins - light sensitive receptors - that regulate the cells’ metabolism. Within the visible light spectrum, ~ 40% is in the blue/green wavelength range and 1–5% of this may reach the subcutaneous adipose tissue.
White (WAT) and brown (BAT) adipose tissues store/release energy and generate heat, respectively. Opsin 3 (encephalopsin) and Opsin 4 (melanopsin) are GPCRs [G protein-coupled receptor] activated by blue light. Opsin 3 signals to regulate the conversion of white into thermogenic beige (Brown-in-white) adipocytes. It is expressed in adipose tissues and may mediate non-visual photoreceptive actions.
Mice in which the Opn3 gene is knocked out are more prone to diet-induced obesity and insulin resistance. The importance of BAT in the metabolic role of Opn3 is indicated by knockout mice presenting with impaired maximum thermogenic capacity along with lower levels of heat production in response to norepinephrine. In vivo light exposure of mice revealed that white light increases brown fat energy expenditure supporting a thermogenic role for OPN3. [see my past Hibernation newsletter for more info]
Opsin 4 was found in both 3T3-L1 adipocytes and human subcutaneous WAT. This receptor mediates the effects of blue light exposure of adipocytes causing increased rate of basal lipolysis and reduced lipid droplet size through coupling to transient receptor potential canonical cation channels.

Opsin 5 (neuropsin), which is sensitive to visible violet light can act to suppress thermogenesis. But unlike OPN3, OPN5 acts via glutamatergic neurons in the hypothalamic preoptic area.
And OPN4 does not only reside in fat, it seems to affect blood vessels and flow, particularly in the context of photorelaxation. The vasorelaxation is wavelength-specific, with a maximal response at ∼430–460 nm. Photorelaxation does not involve endothelial-, nitric oxide-, carbon monoxide-, or cytochrome p450-derived vasoactive prostanoid signaling but is associated with vascular hyperpolarization, as shown by intracellular membrane potential measurements. Blue light (455 nM) can regulate tail artery vasoreactivity ex vivo and tail blood blood flow in vivo - is that why rats bask in the sun? To get ‘chill’ ?
Vitamin D Skin Production loves blue too.
Vitamin D is a hot topic these days. And it needs cool blue color light too.
The pathway begins with a cholesterol molecule, provitamin D, (7-dehydrocholesterol, 7DHC) held within the cell membrane in the skin. When exposed to UVB 290 - 315nm, it is converted very rapidly to previtamin D3.
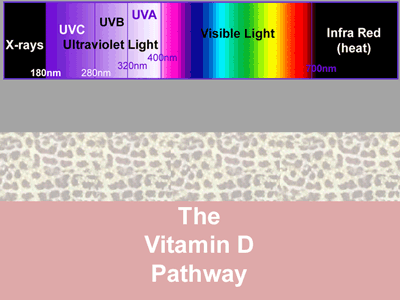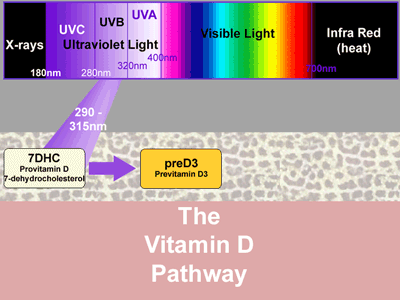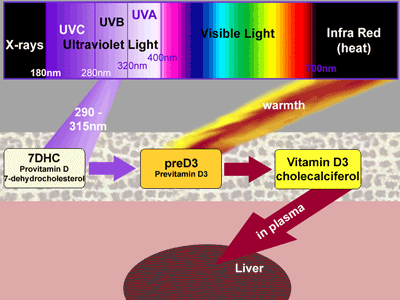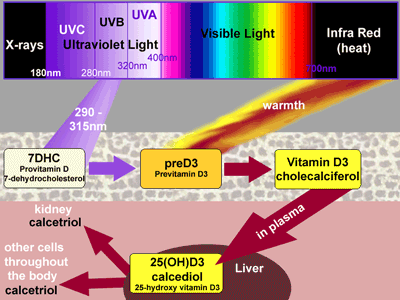
Previtamin D3 is then isomerised slowly, in warm skin, over several hours, to vitamin D3, which is then released and is taken up by a vitamin D-binding protein in the plasma. Carried to the liver, it is hydroxylated into calcediol, 25-hydroxy-vitamin D3.
Calcediol is then circulated back in the bloodstream. In the kidneys, some is converted to the active hormone calcetriol. Calcediol has a vital part to play in the normal functioning of other organs. It is taken up by cells throughout the body, and converted intracellularly to calcetriol then providing local actions upon — the immune system, the cardiovascular system, and control of cell division. Skin cells bathed in sunlight may actually complete the entire pathway from provitamin D to calcetriol all intracellularly, perhaps providing resistance to cancer. However, there are inbuilt safety mechanisms preventing overproduction of vitamin D in the skin. These safeguards, too, rely upon ultraviolet light. [see animated diagram.]
In full sunlight, previtamin D3 is produced very rapidly and accumulates in the skin. Its conversion to vitamin D3 is a much slower, heat dependent process. Because preD3 is also sensitive to ultraviolet light up to 325nm, a proportion is converted quite rapidly into two biologically inactive products, lumisterol3 and tachysterol3. These also accumulate in the skin. However, should excess vitamin D3 build up in the skin, ultraviolet light breaks this down, as well, into three new substances: two suprasterols and 5,6 trans-vitamin D. This latter product does have some biological activity; the others are believed to be inert.
Melatonin production and circadian rhythms require seeing the light
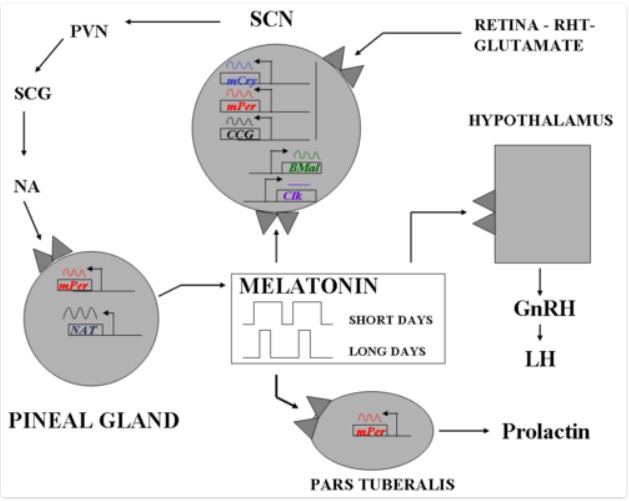
Pineal glands are also a trending topic, especially among the alternative health group. It is a ‘seat of the soul’ and may be the ‘third eye’ , a portal to dream worlds. In the Endocrine world, it is a biologic clock, responsive to light, and producer of melatonin, the Darkness Hormone.

Melatonin production rhythm is generated by interacting networks of clock genes in the bilateral SCN [SupraChiasmaticNucleus]. The SCN rhythm is synchronized to 24 hours mainly by the light-dark cycle acting via the retina and the retinohypothalamic projection to the SCN; the longer the night, the longer the duration of secretion is, and the ocular light signal serves to synchronize the rhythm to 24h and to suppress secretion at the end of the dark phase. A single daily light pulse of suitable intensity and duration in otherwise constant darkness is enough to phase shift and to synchronize the melatonin rhythm to 24h. In humans, intensities of 2500 lux full spectrum light or light preferably in the blue range (460 to 480 nm) are required to fully suppress melatonin at night and shift the rhythm; but lower intensities < 200 lux might partially suppress secretion. The degree of light perception between individuals relates to circadian desynchrony; for example, cortically blind subjects, but with intact unconscious light perception, show abnormally synchronized melatonin and other circadian rhythms. Some blind subjects retain an intact retinohypothalamic tract and therefore can continue to have a normal melatonin response despite lacking conscious light perception. They are the basic structures needed to perceive and transduce non-visual effects of light, and to generate the melatonin rhythm by a closed-loop negative feedback.
Well … this perusal of the blues, turned out to be sunny, not S.A.D. I, for one, cannot wait for spring to come.
“Keep your face always toward the sunshine - and shadows will fall behind you.” ― Walt Whitman
REFERENCES
Sunlight And Fat Metabolism: A New Discovery. Podcast with Peter Light https://blog.humanos.me/fat-tissue-has-receptors-for-sunlight-and-they-affect-fat-metabolism-guest-professor-peter-light/
O N Ekechukwu, M Christian 2021 Metabolic responses of light and taste receptors – unexpected actions of GPCRs in adipocytes. Reviews in Endocrine and Metabolic Disorders https://doi.org/10.1007/s11154-021-09667-9
Ely Contreras et al December 2021 Melanopsin phototransduction: beyond canonical cascades Journal of Experimental Biology 224(23) https://www.researchgate.net/publication/356621012_Melanopsin_phototransduction_beyond_canonical_cascades
Gautam Sikkaa et al. Melanopsin mediates light-dependent relaxation in blood vessels. PNAS | December 16, 2014 | vol. 111 | no. 50 | 17977–17982 www.pnas.org/cgi/doi/10.1073/pnas.1420258111
Vitamin D and Ultraviolet Light - a remarkable process http://www.uvguide.co.uk/vitdpathway.htm
Anna Aulinas. Physiology of the Pineal Gland and Melatonin December 10, 2019. https://www.ncbi.nlm.nih.gov/books/NBK550972/
Hanan Awad Alkozi. Melatonin and Melanopsin in the Eye: Friends or Foes? April 2019 Anales de la Real Academia Nacional de Farmacia 85(1):49-59 https://www.researchgate.net/publication/333354634_Melatonin_and_Melanopsin_in_the_Eye_Friends_or_Foes/






Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults
Timothy M. Brown George C. Brainard ... Kenneth P. Wright Jr https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3001571
sunscreen newsletter https://biomedworks.substack.com/p/nanoparticles-in-the-sunscreen-zinc