Connecting the dots in Connective Tissue research
The Triad of Diseases that correlates with gene mutation.
Ehlers-Danlos Syndrome (EDS), Postural Orthostatic Tachycardia Syndrome (POTS), and Mast Cell Activation Syndrome (MCAS)—a triad of diseases. A recent study found a genetic mutation that links all three conditions.
What is EDS?
Ehlers-Danlos Syndrome refers to a group of genetic conditions that affects the human body’s connective tissue. These syndromes can cause a variety of symptoms to develop, which may be mild in many cases, but can also lead to complications that might become life-threatening. The most common condition is Hypermobility EDS type III (may be up to 1 in 20 people) and it still does not have a genetic marker identified, though it seems to be dominantly inherited.
What is POTS?
Persons with EDS have stretchy tissue and this includes the vascular system. When the cardiac pressure pulse hits their walls with low resistance, the wave is dissipated and blood get trapped in the lower extremities, rather than returning to the heart. Viola, it creates an apparent low volume scenario. Treatment usually is with vasoconstrictors ( eg. pseudoephedrine) and salt loading.
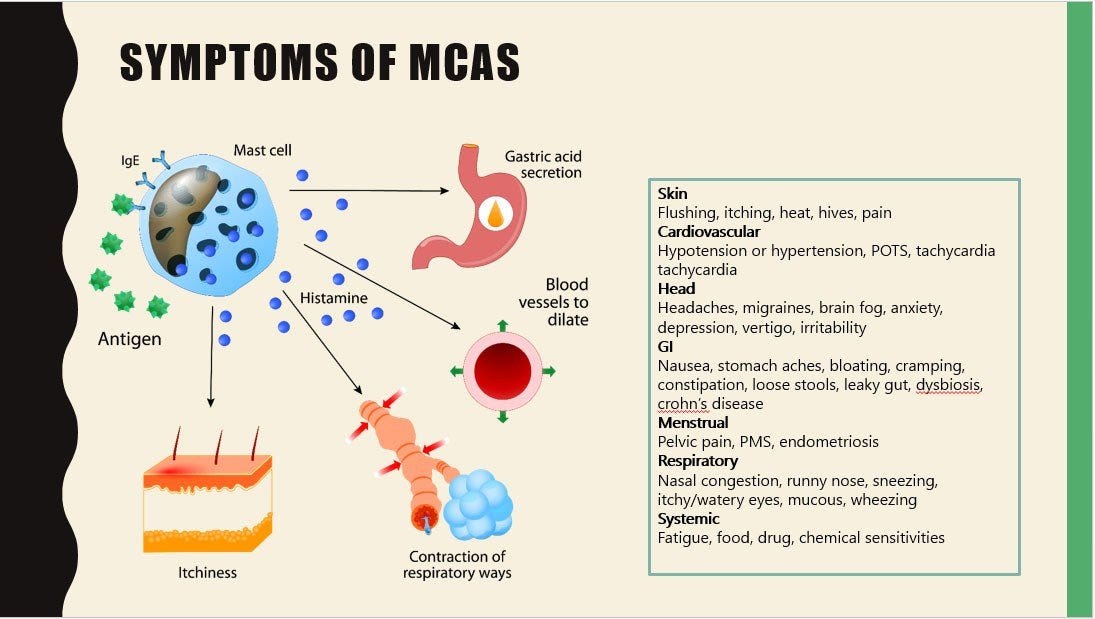
What is MCAS?
Mast Cell Activation Syndrome occurs when the body’s mast cells are easily triggered to degranulate, setting off a cascade of symptoms. It usually is diagnosed by history, symptoms and signs but increased blood level of tryptase (a released component of mast cell) is a diagnostic biomarker. Usual treatment are the mast cell stabilizers such as cromolyn and quercetin.
Darwinian Directive?
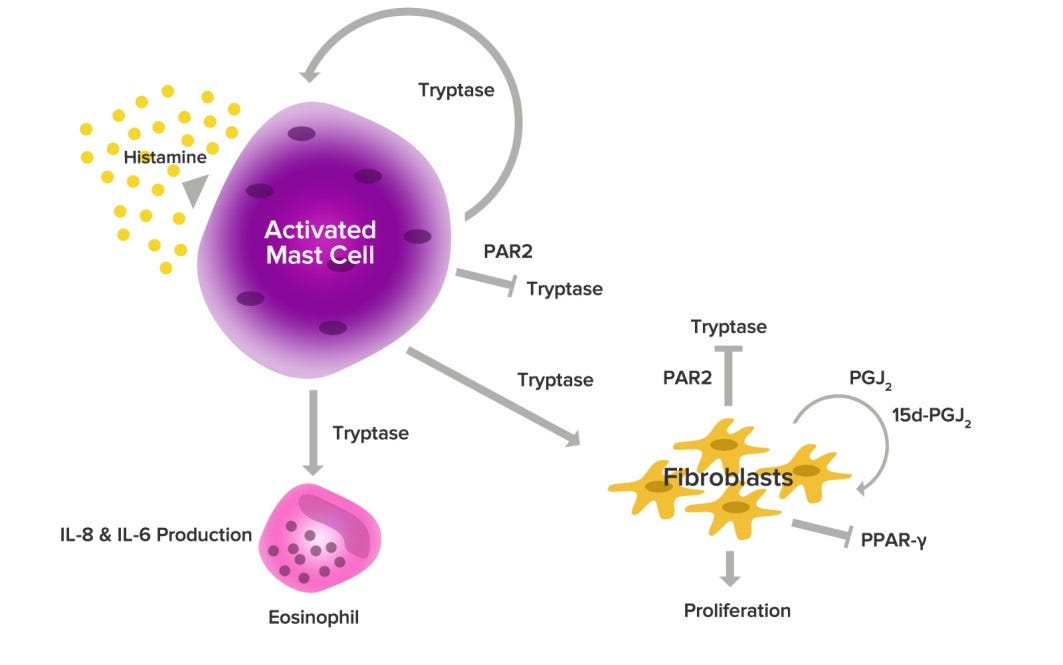
And the genetic mutation that correlates with all three conditions: tryptase gene TPSAB1. In a study involving 96 people with EDS-HT and mast cell issues, POTS symptoms were common. The study participants had another thing in common: higher-than-normal levels of a protein called tryptase in their blood. That subset had a unique genetic signature: an extra copy of a gene called TPSAB1, which makes alpha-tryptase. Researchers then went back through thousands of patient records of healthy people. Those that had high tryptase levels, also had the TPSAB1 mutation. The scientists then interviewed them and found that all of them were living with symptoms similar to those of EDS-HT, POTS, and MCAS.
Can there be a mechanism of action to explain the symptoms and signs? Tryptase is usually released from mast cells upon activation. It is credited with protecting us from snake venoms, so perhaps evolution enriched its genes in the surviving gene pool. Since there is no apparent decrease in expected lifetime with EDS type III, an advantage granted to survive lethal encounters with slithery beasties in the savanna could outweigh the discomforts that come along with the mutation. Of course, connective tissue involves fibroblasts and tryptase is a known stimulator of their proliferation. Perhaps, stretchy tissue needs a lot more stimulation to grow enough connections.
And could tryptase be a therapeutic target? Here is provocative paper proposing just that:
Tryptase, the major secretory product of human mast cells, is emerging as a new target for therapeutic intervention in allergic airways disease. We have investigated the ability of tryptase and inhibitors of tryptase to modulate histamine release from human lung mast cells and have examined the potential contribution of proteinase-activated receptor 2
(PAR2). The tryptase inhibitor APC366 [N-(1-hydroxy-2-naphthoyl)- L -arginyl- L -prolinamide hydrochloride] was highly effective at inhibiting histamine release stimulated by anti-IgE antibody or calcium ionophore from enzymatically dispersed human lung cells. A concentration of APC366 as low as 10 uM was able to inhibit anti-IgE-dependent histamine
release by some 50%. Addition of leupeptin or the tryptic substrate N-benzoyl- D , L -arginine-p-nitroanilide also inhibited IgE-dependent histamine release. Purified tryptase in the presence of heparin stimulated a small but significant release of histamine from lung cells, suggesting that tryptase may provide an amplification signal from activated cells that may be susceptible to proteinase inhibitors. Trypsin was also able to induce histamine release apparently by a catalytic mechanism. Moreover, pretreatment of cells with metabolic inhibitors or with pertussis toxin reduced responses, indicating a noncytoxic pertussis toxin-sensitive G protein-mediated signaling process. Addition to cells of the PAR2 agonists SLIGKV-NH 2 or tc-LIGRLO-NH 2 or appropriate control peptides were without effect on histamine release, and PAR2 was not detected by immunohistochemistry in tissue mast cells. The potent actions of tryptase inhibitors as mast cell-stabilizing agents could be of value in the treatment of allergic inflammation of the respiratory tract, possibly by targeting the non-PAR2-mediated actions of tryptase.
REFERENCES
J Lyons, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet 48, 1564–1569 (2016). https://doi.org/10.1038/ng.3696
SL Seneviratne et al. 2017. Mast cell disorders in Ehlers–Danlos
syndrome. Am J Med Genet Part C Semin Med Genet 175C:226–236. https://www.ehlers-danlos.com/wp-content/uploads/2017-American_Journal_of_Medical_Genetics_Part_C__Seminars_in_Medical_Genetics-20.pdf
JJ Lyons et al. Mendelian inheritance of elevated serum tryptase associated
with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014 May ; 133(5): 1471–1474. doi:10.1016/j.jaci.2013.11.039
A Monaco et al. Association of mast-cell-related conditions with hypermobile syndromes: a review of the literature. Immunol Res. 2022; 70(4): 419–431. doi: 10.1007/s12026-022-09280-1
Mast Cells and Snake Venoms /biomedworks.substack.com/p/mast-cells-and-snake-venoms
E Duchesne et al. Mast cell tryptase stimulates myoblast proliferation; a mechanism relying on protease-activated receptor-2 and cyclooxygenase-2. BMC Musculoskelet Disord 12, 235 (2011). https://doi.org/10.1186/1471-2474-12-235. DOI: 10.1186/1471-2474-12-235
Shaoheng He, et al. Inhibitors of Tryptase as Mast Cell-Stabilizing Agents in the Human Airways: Effects of Tryptase and Other Agonists of Proteinase-Activated Receptor 2 on Histamine Release. Journal of Pharmacology and Experimental Therapeutics April 2004, 309 (1) 119-126; DOI: https://doi.org/10.1124/jpet.103.061291






Could a vitamin deficiency cause 'double-jointedness' and hypermobile Ehlers-Danlos syndrome?
Jacques Courseault et al, Folate-dependent hypermobility syndrome: A proposed mechanism and diagnosis, Heliyon (2023). DOI: 10.1016/j.heliyon.2023.e15387
https://medicalxpress.com/news/2023-04-vitamin-deficiency-double-jointedness-hypermobile-ehlers-danlos.html