My past newsletter discussed using injections of tolerance-inducing formulations to address respiratory allergies, such as hay fever:
Taking Allergy Shots: 'Tis the season for lots of sneezing.
“Well, never fear, the Allergy Specialists have a therapy for you. Desensitization - a series of shots into your deltoid. If you receive multiple small doses of the triggering substance over time, perhaps the allergic response will not be released. That is called … immunosuppression.
This short video is instructive:”
But what about food allergies? In particular, reactions to exposure to nuts. One of my favorite foods is Pad Thai, which is chock full of peanuts. And peanut butter cookies …. mmmm so good.
A recent report brings encouraging news about therapies to induce tolerance to this yummy food. The approach, known as oral immunotherapy, has seen success in trials in infants and children. In 2020, the FDA approved Peanut Allergen Powder (AR101, Palforzia) for use in children, the first licensed oral immunotherapy (OIT) formulation for the treatment of peanut allergy. OIT involves daily ingestion of a food [e.g. peanut flour] in gradually titrating up to increased doses, to induce allergen desensitization and immunomodulation.
Now we can report results from a clinical trial to test whether adults who are allergic to peanuts can also be desensitized. The Grown Up Peanut Immunotherapy (GUPI) trial is the first study entirely in adults with severe allergy to test whether daily doses of peanuts taken under strict supervision can be safely tolerated. Two thirds of the cohort consuming the equivalent of five peanuts without reacting. The average tolerated dose of peanuts increased 100-fold over the course of the trial.
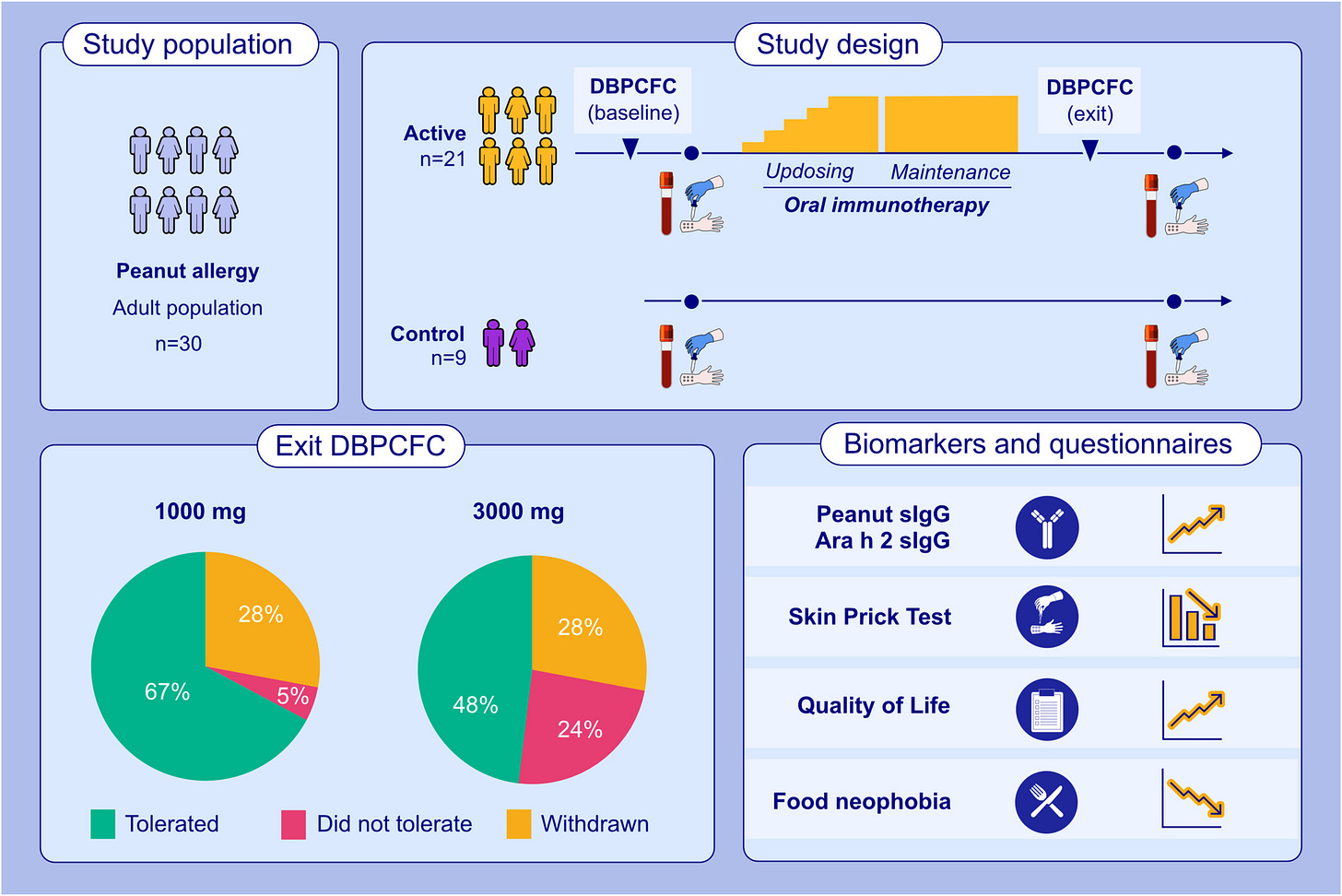
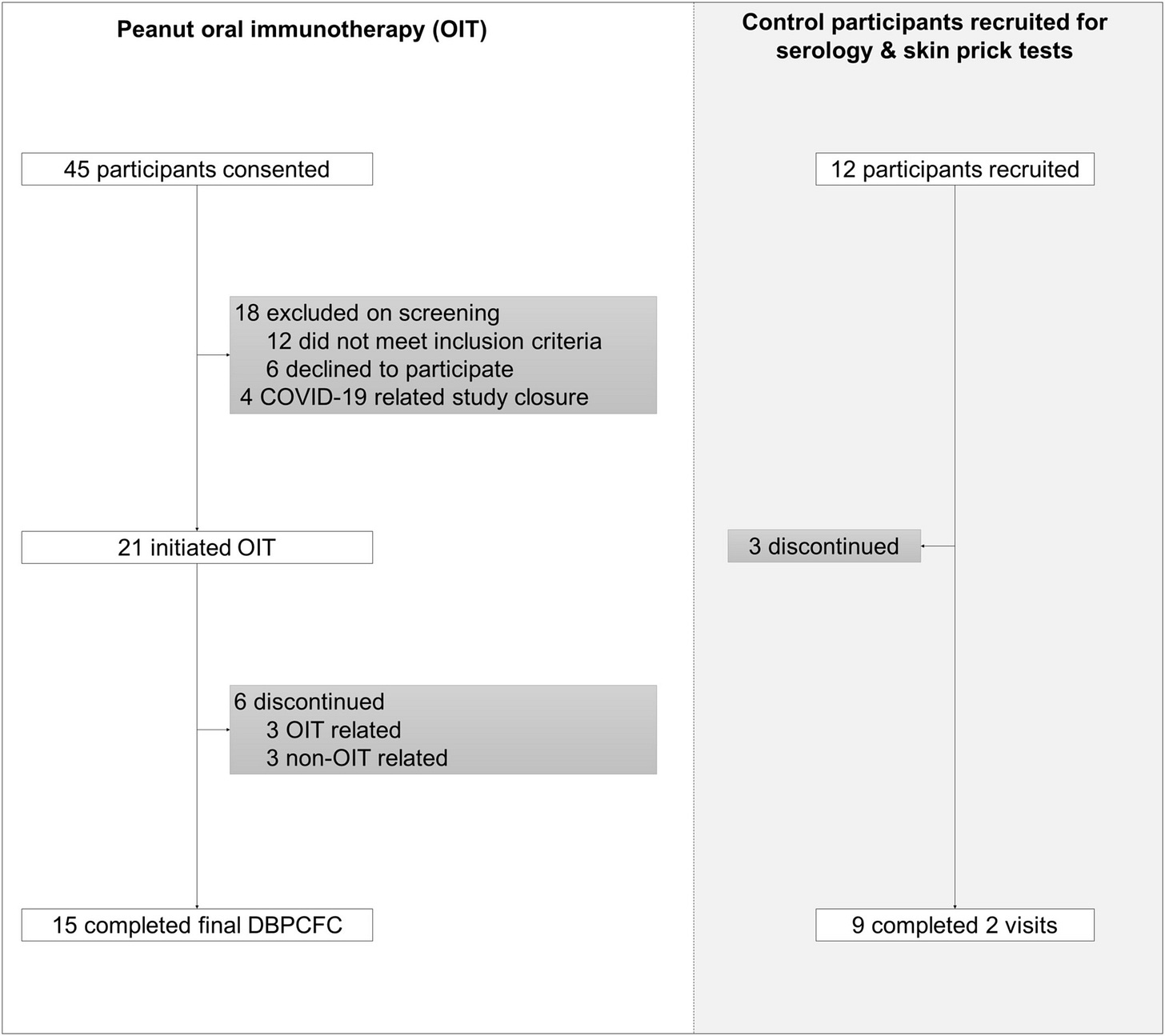
The Phase II trial recruited 21 adults [18 - 40y] with a clinical diagnosis of peanut allergy confirmed via a skin prick test, blood test and then an oral food challenge.
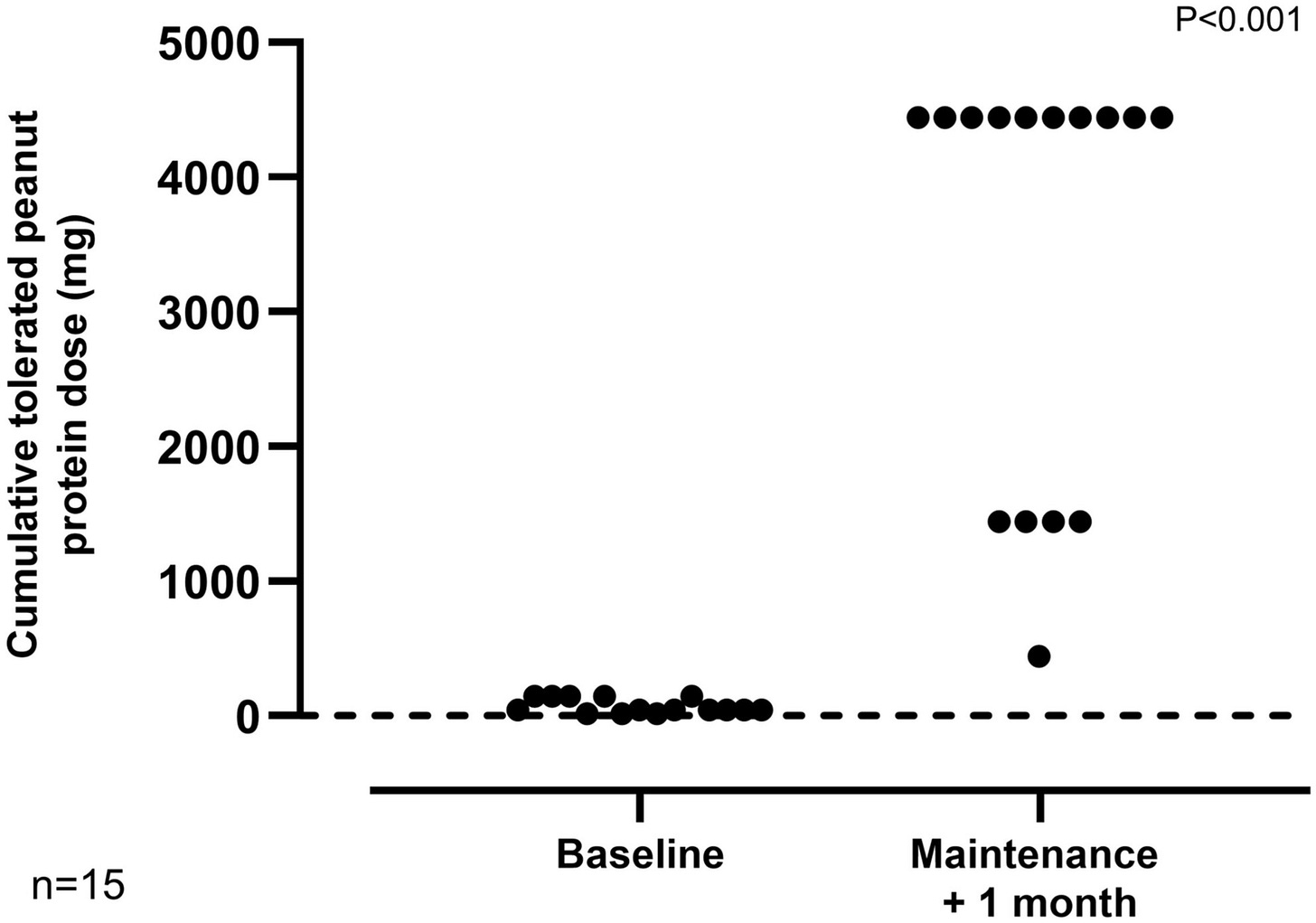
Methods: This phase II trial evaluated peanut OIT in peanut-allergic adults using real-world peanut products. A Simon's mini-max two-stage design, incorporating a stop:go for futility, was employed. A separate untreated control group was also recruited for comparison of mechanistic parameters. Participants underwent baseline double-blind placebo-control food challenges (DBPCFC) with peanut protein doses of 0.3 to 300 mg. Reacting participants were initiated on daily OIT with 2-weekly updosing until reaching a maintenance dose of 1000 mg (four large peanuts). The primary outcome was the proportion of OIT participants who tolerated a cumulative dose of 1.4 g peanut protein during exit DBPCFC (doses provided 0.3-3000 mg). Results: Twenty-one adults (8 female; mean age 24.2 years [SD 4.9]) were enrolled in the OIT group, with 67% achieving the daily maintenance dose and meeting the primary endpoint. Three withdrew due to adverse reactions, and a further three did not complete the trial for reasons unrelated to OIT. The median tolerated dose increased from 30 mg (equivalent to approximately 1/8th of a peanut) to 3000 mg (12 peanuts) at the exit challenge, representing a 100- fold increase (p < 0.0001). OIT was associated with an improvement in QoL measures. Suppression of peanut skin prick test sizes and induction of peanut- specific IgG were observed in OIT but not in control participants.
Grown Up Peanut Immunotherapy (GUPI) study; ClinicalTrials.gov identifier: NCT03648320
- Hannah Hunter et al
Final Results demonstrated that 67% of participants were able to consume at least 1.4g peanut protein [~five peanuts] without reacting. Participants went on to consume peanuts every day at home to maintain their desensitized state.
REFERENCES
H Hunter, et al. Oral Immunotherapy in Peanut‐Allergic Adults Using Real‐World Materials. Allergy (23 April 2025). https://doi.org/10.1111/all.16493