Points to ponder on placenta and pre-eclampsia
Tau proteins, gut bugs, immune receptors and fetal hormones play important roles.
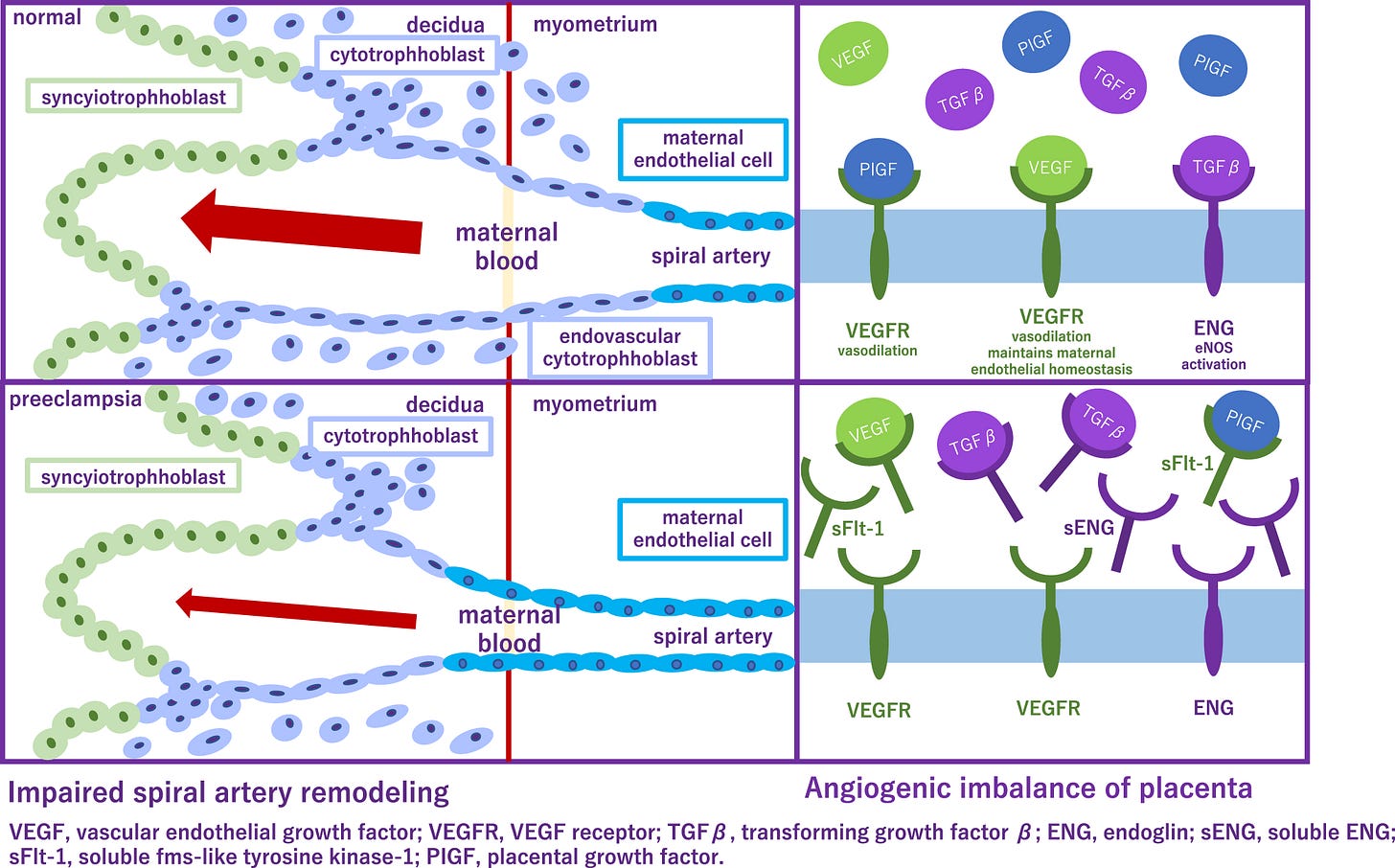
Preeclampsia incidence is 1/15 pregnancies and is the leading cause of maternal and fetal mortality. In this condition, placental blood vessels fail to dilate enough to accommodate the increased blood flow to the fetus. Several recent studies elucidate factors impinging upon that therapeutic target.
Tau Protein
Researchers identified the toxic protein, cis P-tau in the blood and placenta of preeclampsia patients. This misfolded form, cis P-tau, promotes preeclampsia and serves as a biomarker for the condition. It may be used for early diagnosis and is a crucial therapeutic target.
In mice studies, the antibody to cis P-tau efficiently depleted the toxic protein from the blood and placenta, and corrected all features associated with preeclampsia. Clinical features of preeclampsia, including elevated blood pressure, proteinuria and fetal growth restriction, were eliminated.
Preeclampsia (PE) is the leading cause of maternal and fetal mortality globally and may trigger dementia later in life in mothers and their offspring. However, the etiological drivers remain elusive. Cis P-tau is an early etiological driver and blood biomarker in pre-clinical Alzheimer’s and after vascular or traumatic brain injury, which can be targeted by stereo-specific antibody, with clinical trials ongoing. Here we find significant cis P-tau in the placenta and serum of PE patients, and in primary human trophoblasts exposed to hypoxia or sera from PE patients due to Pin1 inactivation. Depletion of cisP-tau from PE patient sera by the antibody prevents their ability to disrupt trophoblast invasion and endovascular activity and to cause the PE-like pathological and clinical features in pregnant humanized tau mice. Our studies uncover that cisP-tau is a central circulating etiological driver and its stereo-specific antibody is valuable for early PE diagnosis and treatment. - S Jash, et al.
Microbiome
Here’s one more study implicating the crucial importance of Mom’s gut bugs in forming a healthy placenta and successful pregnancy:
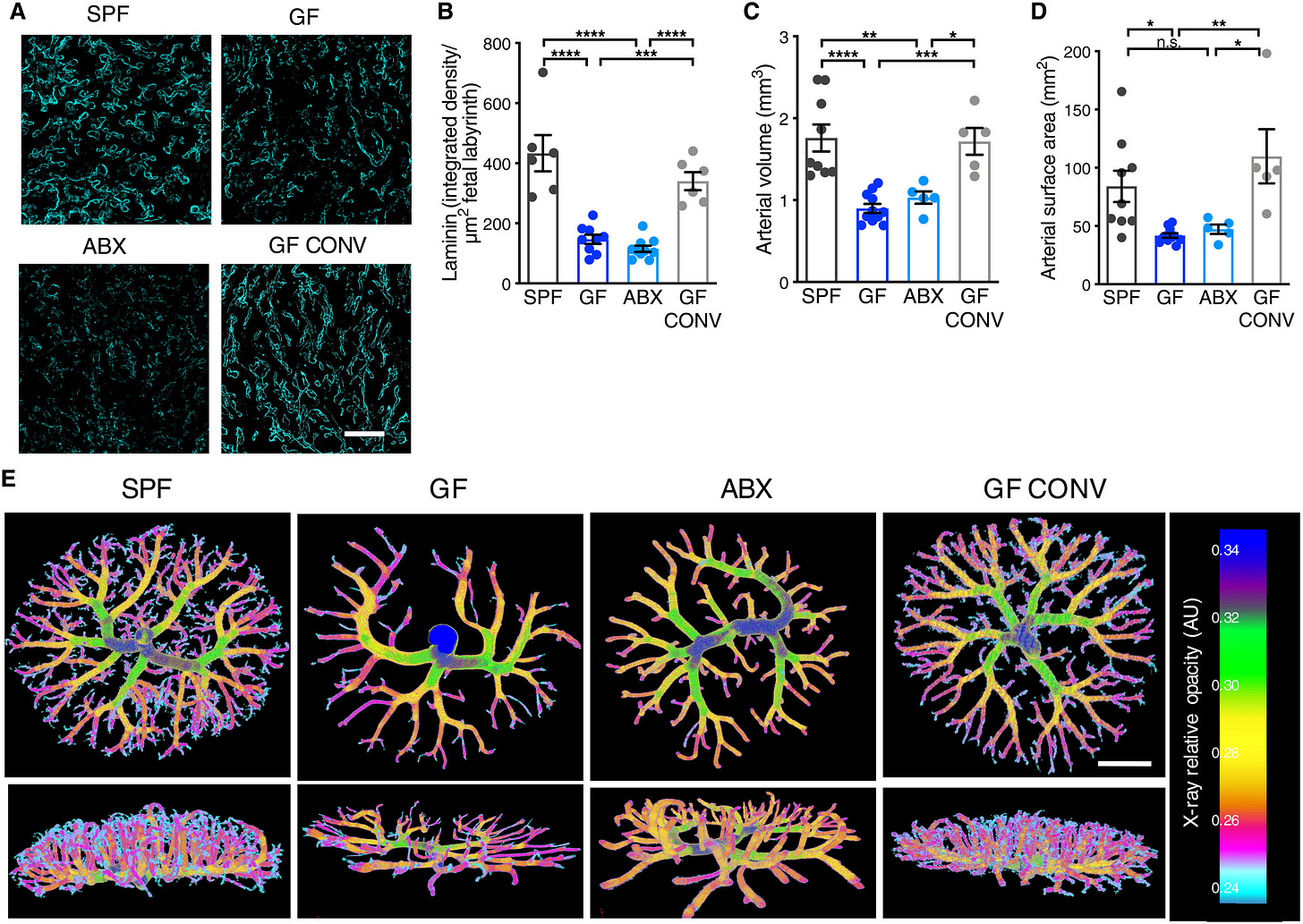
The maternal microbiome is an important regulator of gestational health, but how it affects the placenta as the interface between mother and fetus remains unexplored. Here, we show that the maternal gut microbiota supports placental development in mice. Depletion of the maternal gut microbiota restricts placental growth and impairs feto-placental vascularization. The maternal gut microbiota modulates metabolites in the maternal and fetal circulation. Short-chain fatty acids (SCFAs) stimulate cultured endothelial cell tube formation and prevent abnormalities in placental vascularization in microbiota-deficient mice. Furthermore, in a model of maternal malnutrition, gestational supplementation with SCFAs prevents placental growth restriction and vascular insufficiency. These findings highlight the importance of host-microbial symbioses during pregnancy and reveal that the maternal gut microbiome promotes placental growth and vascularization in mice. - GN Pronovost, et al
Immune Placental Development
A human fetus incorporates the genetic material from both parents; thus it is foreign to the pregnant mom’s body. The immune system must make adjustments that balance a tolerance for the developing fetus with protection against harmful foreign insults such as infections.
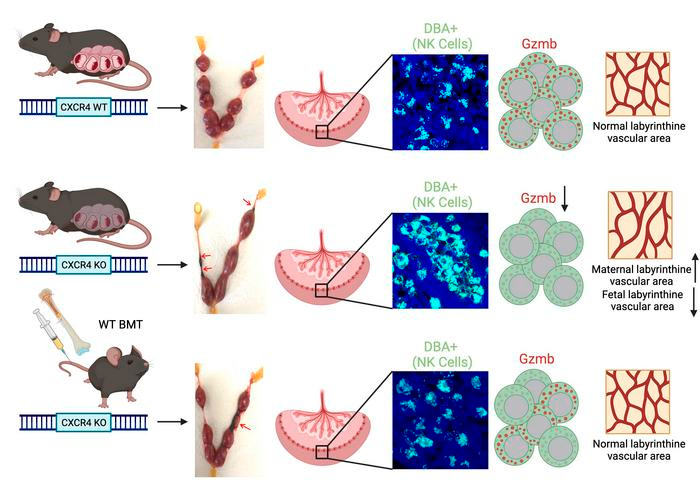
Many of these adaptations occur within the decidua — ie tissue that surrounds and interfaces with the placenta. A protein called C-X-C chemokine receptor type 4 (CXCR4), and its ligand are critical for trafficking bone marrow cells in organs and tissues around the body. CXCR4 is also expressed in higher amounts in the uterus at the beginning of pregnancy. However, it is not the CXCR4 expression within the uterine cells themselves, but rather the CXCR4 expression from outside of the uterus, that is important in pregnancy maintenance. In early pregnancy these natural killer cells in the decidua are important for tissue and blood vessel remodeling requisite for the placenta to properly develop and for the fetus to receive nutrients and oxygen from the parent.
CXCR4 is a key regulator of the development of Natural Killer (NK) cells and dendritic cells, both of which play an important role in early placental development and immune tolerance at the maternal-fetal interface. However, the role of CXCR4 in pregnancy is not well understood. Our study demonstrates that adult-induced global genetic CXCR4 deletion, but not uterine-specific CXCR4 deletion, was associated with increased pregnancy resorptions and decreased litter size. CXCR4-deficient mice had decreased NK cells and increased granulocytes in the decidua, and increased leukocyte numbers in peripheral blood. We found that CXCR4-deficient mice had abnormal decidual NK cell aggregates and NK cell infiltration into trophoblast areas beyond the giant cell layer. This was associated with low NK cell expression of granzyme B, a NK cell granule effector, indicative of NK cell dysfunction. Pregnancy failure in these mice was associated with abnormalities in placental vascular development and increased placental expression of inflammatory genes. Importantly, adoptive bone marrow transfer of wild type CXCR4+ bone marrow cells into CXCR4-deficient mice rescued the reproductive deficits by normalizing NK cell function and mediating normal placental vascular development. Collectively, our study found an important role for maternal CXCR4 expression in immune cell function, placental development and pregnancy maintenance. - Fang Lyu, et al.
Fetal Hormones
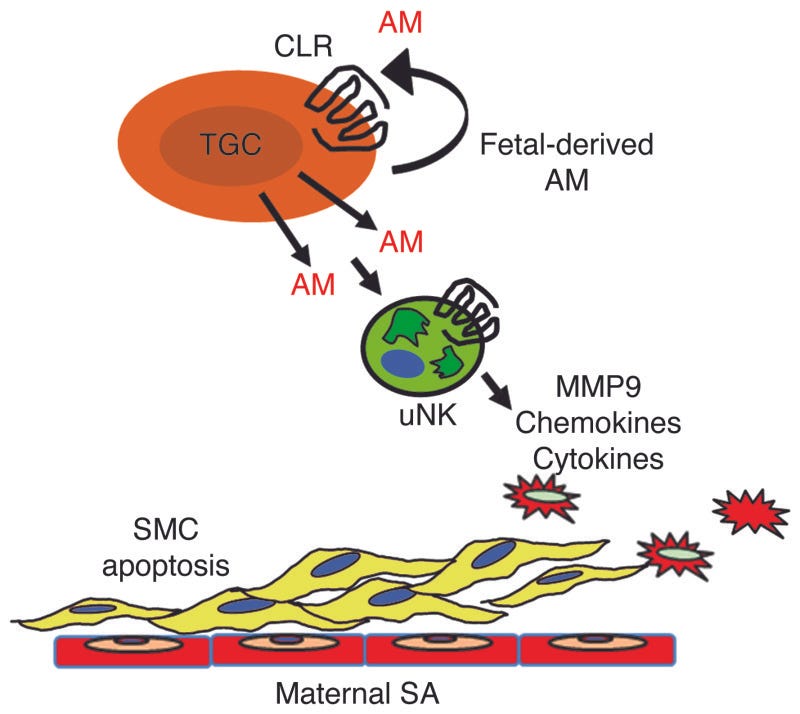
In another mouse experiment, researchers determined that the hormone, adrenomedullin, plays a crucial role in preventing the pregnancy complication preeclampsia. When secreted by the fetus [not the mother] at critical times during the pregnancy, it can protect the moms from preeclampsia. In a normal pregnancy, the fetus secretes adrenomedullin into the placenta during the second trimester, signaling "natural killer cells" to help dilate the mother's blood vessels.
The remodeling of maternal uterine spiral arteries (SAs) is an essential process for ensuring low-resistance, high-capacitance blood flow to the growing fetus. Failure of SAs to remodel is causally associated with preeclampsia, a common and life-threatening complication of pregnancy that is harmful to both mother and fetus. Here, using both loss-of-function and gain-of-function genetic mouse models, we show that expression of the pregnancy-related peptide adrenomedullin (AM) by fetal trophoblast cells is necessary and sufficient to promote appropriate recruitment and activation of maternal uterine NK (uNK) cells to the placenta and ultimately facilitate remodeling of maternal SAs. Placentas that lacked either AM or its receptor exhibited reduced fetal vessel branching in the labyrinth, failed SA remodeling and reendothelialization, and markedly reduced numbers of maternal uNK cells. In contrast, overexpression of AM caused a reversal of these phenotypes with a concomitant increase in uNK cell content in vivo. Moreover, AM dose-dependently stimulated the secretion of numerous chemokines, cytokines, and MMPs from uNK cells, which in turn induced VSMC apoptosis. These data identify an essential function for fetal-derived factors in the maternal vascular adaptation to pregnancy and underscore the importance of exploring AM as a biomarker and therapeutic agent for preeclampsia. - M Li, et al.
Indeed, robust research is going on in this crucial clinical arena. May it make it to the bedside, soon!
REFERENCES
K Bokuda, A Ichihara. Preeclampsia up to date—What’s going on?. Hypertens Res 46, 1900–1907 (2023). https://doi.org/10.1038/s41440-023-01323-w
S Jash, et al. Cis P-tau is a central circulating and placental etiologic driver and therapeutic target of preeclampsia, Nature Communications (2023). DOI: 10.1038/s41467-023-41144-6
GN Pronovost, et al. The maternal microbiome promotes placental development in mice, Science Advances (2023). DOI: 10.1126/sciadv.adk1887
F Lyu, et al. Maternal CXCR4 deletion results in placental defects and pregnancy loss mediated by immune dysregulation, JCI Insight (2023). DOI: 10.1172/jci.insight.172216. https://insight.jci.org/articles/view/172216
M Li, et al. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. Journal of Clinical Investigation, 2013; DOI: 10.1172/JCI67039