One of my past newsletters reported on many modalities that can help alleviate pain and stiffness. It also referred back to my newsletters discussing rehabilitation progress, some using sound vibration devices.
Good Vibrations
The medical truthers who resist all the mandates and refuse to don the face diapers cannot get any care at all in the state of Massachusetts. One must travel to other regions of the USA to seek some relief. These so-called ‘healthcare providers’ who ban those with exposed faces, are really agents acting on behalf of their overlords. And who might tha…
More research reports now detail advances in areas of chronic pain relief and also therapies for spasticity arising from strokes.
Cutaneous vibrotactile stimulation of the hand reduces spastic hypertonia
Engineers developed a glove-like wearable medical device that achieves as good or better results as the injections or drugs by applying simple, high-frequency mechanical vibrations to the hands and fingers.

Cutaneous vibrotactile stimulation of the hand provides significant reductions in spastic hypertonia, as compared to muscle stimulation. Daily stimulation from the VTS Glove can relieve spasticity and hypertonia resulting from brain injuries. In a clinical trial, 50+% of the participants who had regularly used BotulismToxin-A, the VTS Glove provided equal or greater symptom relief.
Pain Relief Paths
Scientists demonstrated the effectiveness of using low-intensity focused ultrasound to modulate the activity in a critical region in the brain that processes and regulates pain signals. Applying low-intensity ultrasound to the dorsal anterior cingulate cortex reduced pain, diminished bodily responses to pain, and decreased pain-related brain activity without the need for invasive procedures
The dorsal anterior cingulate cortex (dACC) is a critical brain area for pain and autonomic processing, making it a promising noninvasive therapeutic target. We leverage the high spatial resolution and deep focal lengths of low-intensity focused ultrasound (LIFU) to noninvasively modulate the dACC for effects on behavioral and cardiac autonomic responses using transient heat pain stimuli. A N = 16 healthy human volunteers (6 M/10 F) received transient contact heat pain during either LIFU to the dACC or Sham stimulation. Continuous electroencephalogram (EEG), electrocardiogram (ECG), and electrodermal response (EDR) were recorded. Outcome measures included pain ratings, heart rate variability, EDR response, blood pressure, and the amplitude of the contact heat-evoked potential (CHEP).
LIFU reduced pain ratings by 1.09 ± 0.20 points relative to Sham. LIFU increased heart rate variability indexed by the standard deviation of normal sinus beats (SDNN), low-frequency (LF) power, and the low-frequency/high-frequency (LF/HF) ratio. There were no effects on the blood pressure or EDR. LIFU resulted in a 38.1% reduction in the P2 CHEP amplitude. Results demonstrate LIFU to the dACC reduces pain and alters autonomic responses to acute heat pain stimuli. This has implications for the causal understanding of human pain and autonomic processing in the dACC and potential future therapeutic options for pain relief and modulation of homeostatic signals. - A Strohman, et al.
Why does rubbing work?
When you whack your thumb with a hammer, why do you feel relief after vigorously shaking it? The mechanism behind this effect is explained by the "gate control theory." Shaking modifies the pain signals sent to the spinal cord in the superficial dorsal horn area; they are transmitted by Aδ and C fibers. This same area is activated by connections for sensing touch (from the Aβ mechanoreceptors) and their crosstalk can block the pain signals.

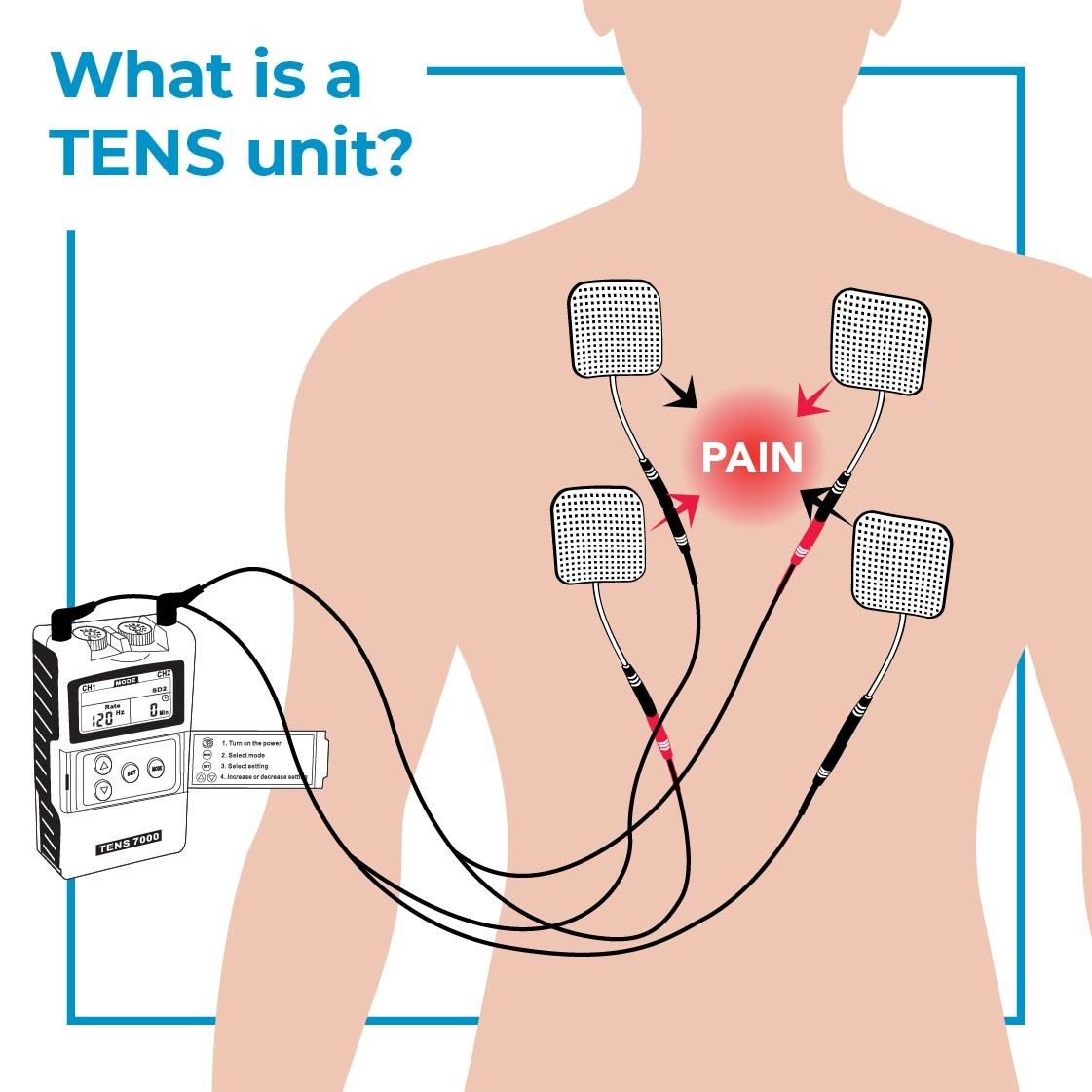
Aβ-LTMRs are further divided into rapidly adapting (RA) and slowly adapting (SA) types based on their firing patterns in response to a sustained mechanical stimulus. At baseline conditions, the co-activation of touch- and pain-sensing neurons inhibits the flow of nociceptive information. Thus massaging a sore area activates Aβ low-threshold mechanoreceptors (Aβ-LTMRs), attenuating the pain. Real-life therapy procedures that activate Aβ-LTMRs include the spinal cord stimulator (SCS), transcutaneous electrical nerve stimulation (TENS), massage therapy and electroacupuncture.
Aβ-LTMRs play globally inhibitory, but locally promoting, roles for the chronic pain situation of mechanical hyperalgesia. If so, then globally activating but locally inhibiting the Aβ-LTMRs would be best for treating it. Best to target peripheral Aβ-LTMRs at least 2-3 dermatomes away from the injury site, with your TENS stimuli.
Looking forward to more ‘earthshaking’ advances in the pain relief arena, as these findings are applied in clinical practice.
REFERENCES
C Seim, et al. Relief of post-stroke spasticity with acute vibrotactile stimulation: controlled crossover study of muscle and skin stimulus methods, Frontiers in Human Neuroscience (2023). https://www.frontiersin.org/articles/10.3389/fnhum.2023.1206027/full
C Seim, et al. Daily Vibrotactile Stimulation Exhibits Equal or Greater Spasticity Relief Than Botulinum Toxin in Stroke, Archives of Physical Medicine and Rehabilitation (2023). https://pubmed.ncbi.nlm.nih.gov/37149017/
A Strohman, et al. Low-intensity focused ultrasound to the human dorsal anterior cingulate attenuates acute pain perception and autonomic responses., The Journal of Neuroscience (2024). DOI: 10.1523/JNEUROSCI.1011-23.2023
M Gautam, et al. Distinct local and global functions of mouse Aβ low-threshold mechanoreceptors in mechanical nociception, Nature Communications (2024). DOI: 10.1038/s41467-024-47245-0