Time again to tell some more “tidbits” of info; this time the focus is bone related. We start with cartilage growth times.
Arthritic cartilage epigenetic clock
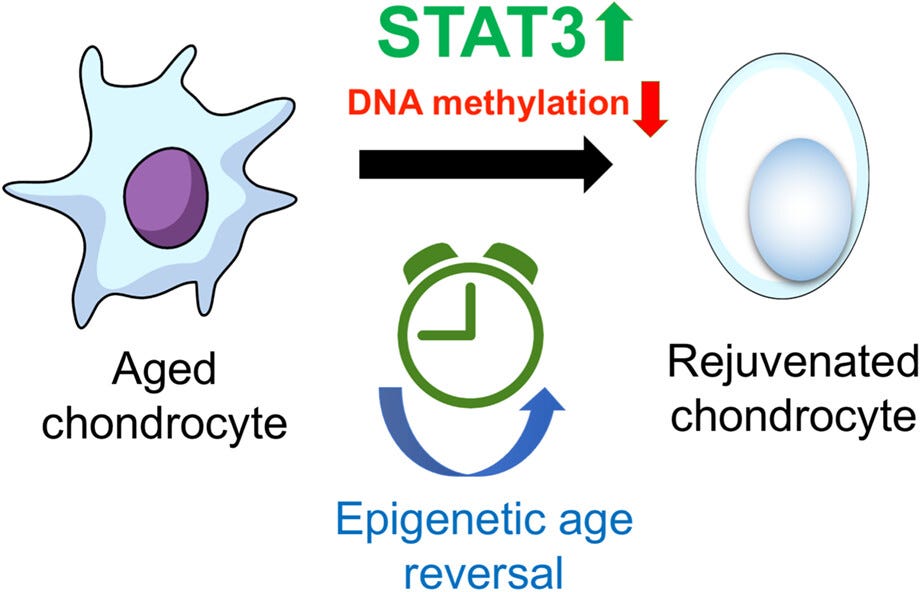
Signal Transducer and Activator of Transcription 3 (STAT3) turns back the aging cartilage of osteoarthritis to a younger vigorous state.
An enzyme called DNA methyltransferase 3 beta (DNMT3B), interacts with STAT3. When STAT3 was inactivated, it triggered DNMT3B to add aging epigenetic methylation to the DNA molecule, and promoted the progression of knee osteoarthritis in injured mice.
However, a continous hyperactivation of the immature program in cartilage cells (induced by IL-6 cytokines) tended to promote inflammation and resulted in degeneration and fibrosis. The new tissue was not tough enough to withstand the arthritic burdens. An optimal balance is sought.
Ankle > knee > hip regenerative capacity
This study describes region-specific regenerative differences in human osteoarthritis cartilage. Varies according to joint locations — distal more labile, proximal much less.
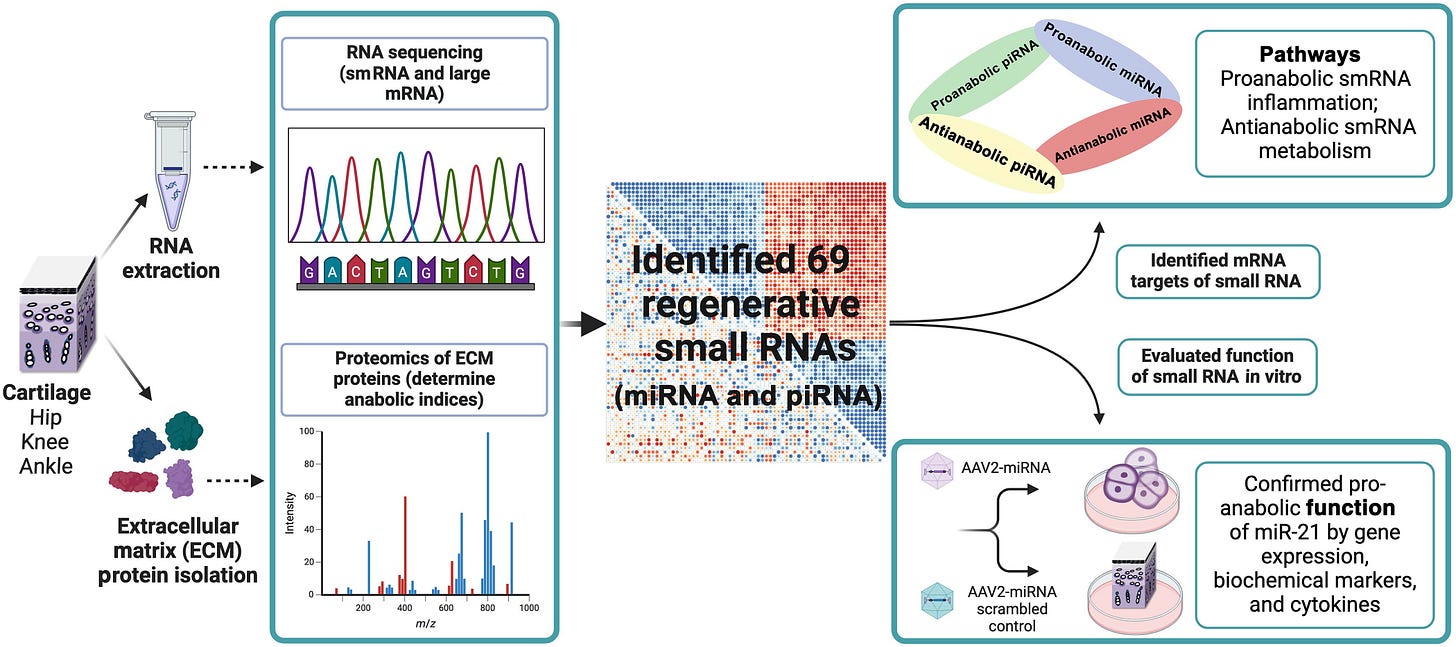
Technology provided a definitive readout of regenerative activity, which identified small RNAs (smRNAs) functioning as master regulators—some becoming activated to promote cartilage repair, while others were silenced because they normally inhibit growth.
Their data reveal a smRNA-regulated anabolic response in OA cartilage that varies by joint site. The ankle's ability to regenerate cartilage uses the same mechanisms similar to those that enable zebrafish and salamanders to regrow severed limbs. When osteoarthritis occurs, these smRNA coordinate to repair cartilage.
These smRNA are in human ankle joints. But they are scarcer in human knees, and even more so in hips. Ankle cartilage’s regenerative environment could serve as a template for enhancing cartilage repair in less regenerative joints. Researchers hope to optimize this innate restorative function and target it to knees and hips, which are the most common sites for osteoarthritis.
Gait retraining to remodel knees
Remodeling arthritic joints may be as simple as adjusting and correcting joint position.
Scientists tested gait analysis and corrective intervention in 68 subjects with mild/moderate knee osteoarthritis. They used MRI scans to track efficacy of treatment. The group who were trained to angle their feet slightly inward or outward from their natural alignment, had slower cartilage degeneration in the inner part of their knee, compared with those who were encouraged to walk more frequently without changing their foot position. This treated group also reduced their pain score by 2.5 points on a 10-point scale. The control group reduced their pain scores by little more than a point.
This study is registered with ClinicalTrials.gov, NCT02767570
So, adjusting foot angle during walking, when tailored to an individual's gait, can reduce knee pain and slow cartilage degeneration in knee osteoarthritis. This personalized, noninvasive approach decreases joint loading by 4%, offers pain relief comparable to over-the-counter medications, and may delay the need for surgery without suffering drug-related side effects.
[see also past newsletter: Memorial Weekend: reporting winners in rehab research for our wounded warriors: If you straighten out, it will grow]
Digit Development
Human fingers and toes do not grow outward; instead, they form from within a larger foundational bud, as intervening cells recede to reveal the digits beneath.
Scientists produced a spatial cell atlas of the entire developing human limb, resolved in space and time. This atlas provides a resource that captures the intricate processes governing the limbs' rapid development during the early stages of limb formation.
“This video shows the dynamic gene expression patterns of IRX1, SOX9 and MSX1, critical genes involved in limb formation. Their distinct distribution ensures the 'chiselling' process takes place. IRX1, crucial for digit formation, and SOX9, essential for skeletal development, converge into five distinct lengths within the developing limb, while MSX1, associated with undifferentiated cells, occupies the interdigital spaces between these clusters. At approximately week seven of development, molecules responsible for interdigital cell death are activated, leading to the elimination of cells in the intervening spaces. This orchestrated cell death finally unveils the well-defined shapes of fingers or toes. Credit: DOI: 10.1038/s41586-023-00000-0”
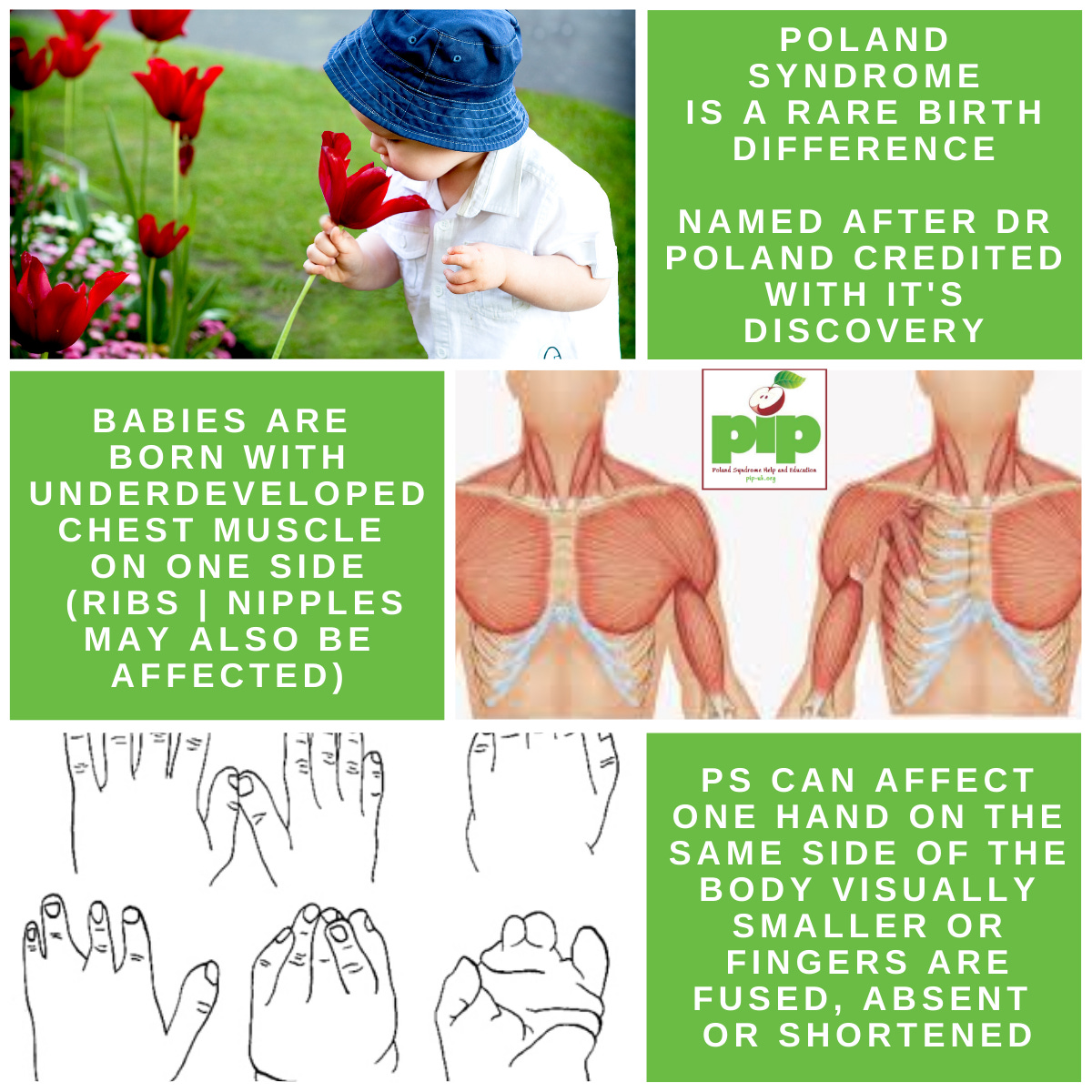
The atlas uncovers possible links between developmental processes and congenital limb syndromes, such as short fingers and extra digits. One such syndrome is described in a past newsletter:
REFERENCES
A Sarkar, et al. STAT3 promotes a youthful epigenetic state in articular chondrocytes, Aging Cell (2023). DOI: 10.1111/acel.13773
Ming-Feng Hsueh, et al. Anabolic indices of matrix proteins identify regenerative small RNA intrinsic to human cartilage. Science Advances (2025). DOI: 10.1126/sciadv.adu8440
SD Uhlrich, et al. Personalised gait retraining for medial compartment knee osteoarthritis: a randomised controlled trial. The Lancet Rheumatology (2025). DOI: 10.1016/S2665-9913(25)00151-1
T Oláh, et al. Axial alignment is a critical regulator of knee osteoarthritis. Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abn0179
H Zhang, et al. A human embryonic limb cell atlas resolved in space and time. Nature (2023). http://dx.doi.org/10.1038/s41586-023-06806-x