My past newsletter reported the great news of the breakdown of waste plastics by bugs found in nature.
Refuse Rescuers: discovering critters who chomp up trash
Been reading about newly discovered microbes and worms that use plastic as a nutritional resource. Touted as the solution to pollution!!
“Plastics - Degradation by insects and their microbiome
In the ideal world, our garbage would break down and be recycled into new life. Such is the life cycle of the tree leaves. But can we hope to do this with the growing mountains of discarded plastics? Yes.
The saliva of Galleria mellonella larvae (wax worms) is capable of oxidizing and depolymerizing polyethylene (PE), the bottleneck step in PE biodegradation, with just hours exposure at room temperature and neutral pH. The saliva contains the enzymes capable of converting polyethylene into ethylene glycol [antifreeze].”
Aspirin / acetylsalicylic acid
Synthetic chemists are now making plastic polymers that naturally hydrolyze into acetylsalicylic acid.
Yes, chemists learned from the bugs and can take that plastic trash and convert it into aspirin. Their own version of “Recycling’.
A group of Japanese chemists came up with a solution to ocean islands of plastic pollution. They created a plastic using acetylsalicylic acid (aspirin). When discarded, the "aspirin plastic" converts back into its starting material -- which is recovered and recycled to make fresh plastic.
The poly(dehydroaspirin) = "aspirin plastic" = does not have to breakdown using bacteria. It can be heated along with an acid (e.g., hydrochloric or sulfuric) and water to result in hydrolysis. This reaction breaks apart the polymer, yielding salicylic acid as a chemical substrate. Once recovered and reacted with acetic anhydride - voila! aspirin.
A vinyl polymer with a cyclic hemiacetal ester skeleton was synthesized via the radical polymerization of 2-methylene-4H-benzo[d][1,3]dioxin-4-one (MBDO; so-called ‘dehydroaspirin’). This material could be decomposed to acetic acid and salicylic acid (the raw ingredients for MBDO) by acid hydrolysis, and thus has potential as a recyclable vinyl polymer. - A Kazamaa, Y Kohsaka.
Acetominophen / paracetamol
Not to be outdone fellow pain killer, acetominophen, joined the recycle route in chemistry.
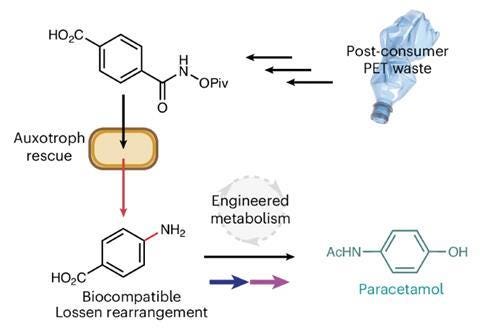
Nature has evolved an exquisite yet limited set of chemical reactions that underpin the function of all living organisms. By contrast, the field of synthetic organic chemistry can access reactivity not observed in nature, and integration of these abiotic reactions within living systems offers an elegant solution to the sustainable synthesis of many industrial chemicals from renewable feedstocks. Here we report a biocompatible Lossen rearrangement that is catalysed by phosphate in the bacterium Escherichia coli for the transformation of activated acyl hydroxamates to primary amine-containing metabolites in living cells. Through auxotroph rescue, we demonstrate how this new-to-nature reaction can be used to control microbial growth and chemistry by generating the essential metabolite para-aminobenzoic acid. The Lossen rearrangement substrate can also be synthesized from polyethylene terephthalate and applied to whole-cell biocatalytic reactions and fermentations generating industrial small molecules (including the drug paracetamol), paving the way for a general strategy to bioremediate and upcycle plastic waste in native and engineered biological systems. - NW Johnson, et al.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Your future is …. plastics!
REFERENCES
A Sanluis-Verdes, et al. 2022 Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. doi: https://doi.org/10.1101/2022.04.08.487620.
A Kazamaa, Y Kohsaka. Radical polymerization of ‘dehydroaspirin’ with the formation of a hemiacetal ester skeleton: a hint for recyclable vinyl polymers. Polym. Chem., 2019,10, 2764-2768. DOI:10.1039/C9PY00474B
NW Johnson, et al. A biocompatible Lossen rearrangement in Escherichia coli. Nature Chemistry (2025). DOI: 10.1038/s41557-025-01845-5






Thank you for this interesting article, which delivers optimism. There is only one little snag. If I am not mistaken the problem with plastics like PVC is not the material itself but the plasticisers and added compounds like flame retardants, which are problematic for our future health. Those compounds are the reason why aged water kettles literally break at some point. Any ideas about phthalat degradation?