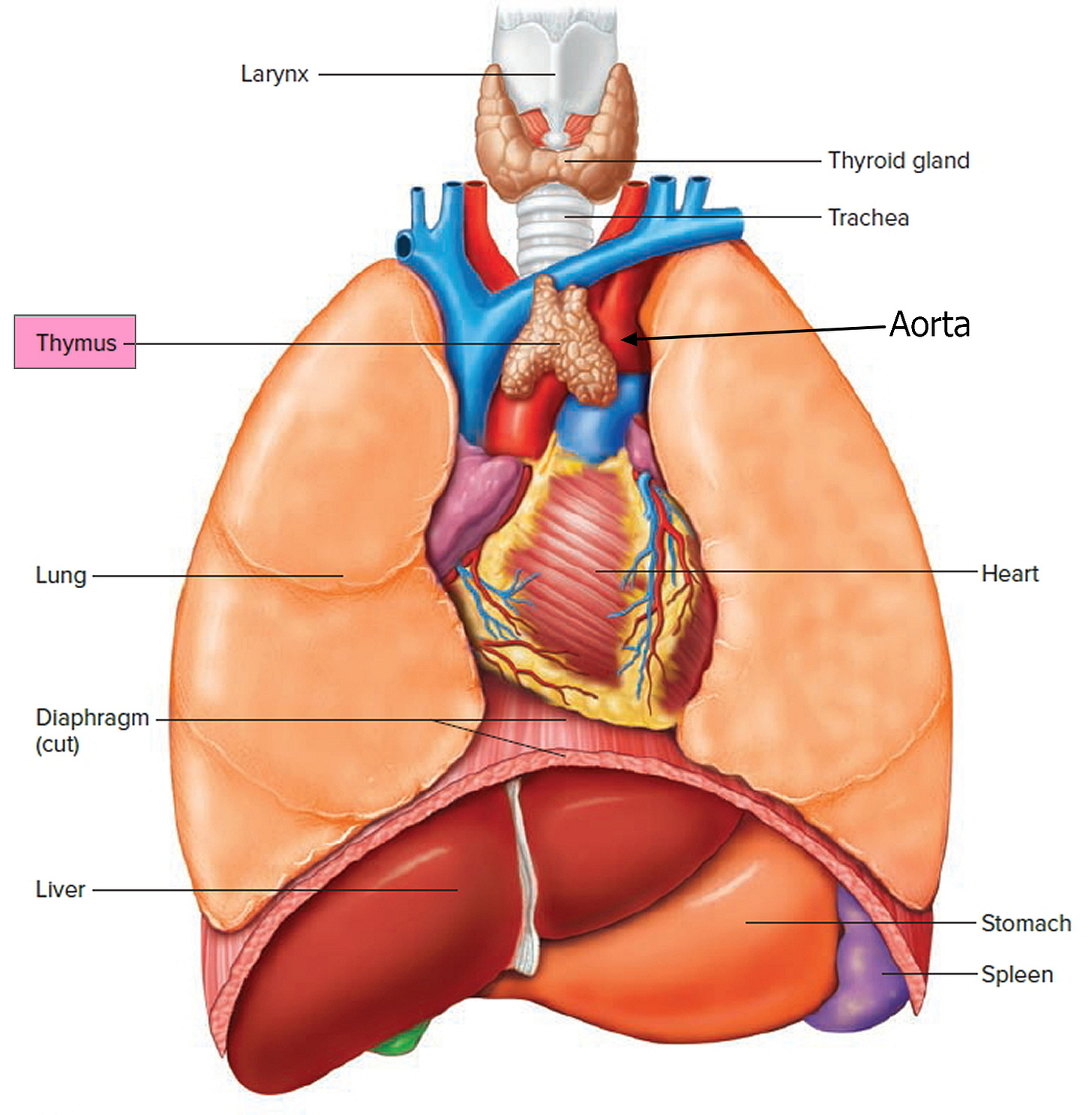
The thymus gland produces and releases thymosin, to create T lymphocytes. It hangs out around the aorta, just below the thyroid gland. Dogma was, that it shaped the immune system’s recognition of self and non-self, during fetal development. Then, apparently, it subsequently becomes irrelevant.
Not so, says new research.
Thymus removal ups risk of death
A recent observational study looked at those who had their thymus removed during surgery, for example, congenital heart defect repair children. Case controls were compared for immune consequences over the long term.
ABSTRACT Background: The function of the thymus in human adults is unclear, and routine removal of the thymus is performed in a variety of surgical procedures. We hypothesized that the adult thymus is needed to sustain immune competence and overall health. Methods: We evaluated the risk of death, cancer, and autoimmune disease among adult patients who had undergone thymectomy as compared with demographically matched controls who had undergone similar cardiothoracic surgery without thymectomy. T-cell production and plasma cytokine levels were also compared in a subgroup of patients. Results: After exclusions, 1420 patients who had undergone thymectomy and 6021 controls were included in the study; 1146 of the patients who had undergone thymectomy had a matched control and were included in the primary cohort. At 5 years after surgery, all-cause mortality was higher in the thymectomy group than in the control group (8.1% vs. 2.8%; relative risk, 2.9; 95% confidence interval [CI], 1.7 to 4.8), as was the risk of cancer (7.4% vs. 3.7%; relative risk, 2.0; 95% CI, 1.3 to 3.2). Although the risk of autoimmune disease did not differ substantially between the groups in the overall primary cohort (relative risk, 1.1; 95% CI, 0.8 to 1.4), a difference was found when patients with preoperative infection, cancer, or autoimmune disease were excluded from the analysis (12.3% vs. 7.9%; relative risk, 1.5; 95% CI, 1.02 to 2.2). In an analysis involving all patients with more than 5 years of follow-up (with or without a matched control), all-cause mortality was higher in the thymectomy group than in the general U.S. population (9.0% vs. 5.2%), as was mortality due to cancer (2.3% vs. 1.5%). In the subgroup of patients in whom T-cell production and plasma cytokine levels were measured (22 in the thymectomy group and 19 in the control group; mean follow-up, 14.2 postoperative years), those who had undergone thymectomy had less new production of CD4+ and CD8+ lymphocytes than controls (mean CD4+ signal joint T-cell receptor excision circle [sjTREC] count, 1451 vs. 526 per microgram of DNA [P=0.009]; mean CD8+ sjTREC count, 1466 vs. 447 per microgram of DNA [P<0.001]) and higher levels of proinflammatory cytokines in the blood. Conclusions. In this study, all-cause mortality and the risk of cancer were higher among patients who had undergone thymectomy than among controls. Thymectomy also appeared be associated with an increased risk of autoimmune disease when patients with preoperative infection, cancer, or autoimmune disease were excluded from the analysis. - KA Kooshesh et al.
Admittedly, there is a bias in the data. Those whose thymus’ were taken out could have been somehow, ‘sicker’ than the ‘matched controls’. But, separate research does support thyroid gland autoimmunity increasing with loss of thymus.
Thymus and pregnancy
The thymus gland performs significant roles in both metabolic control and immunity during pregnancy. It can prevent miscarriage and the development of gestational diabetes. The huge pregnancy-associated increase in estrogens leads to changes in the mother’s thymus, that accommodate the physiological changes that arise. The T-reg lymphocytes migrate to the mother's adipose tissue, preventing inflammation and helping to control glucose levels. RANK, a receptor expressed in the thymus epithelium, is a key molecule involved in this mechanism of action.
The postnatal period is important too. Specific maternal immune cells in breast milk cross the wall of the baby's intestine to enter their own thymus gland. Once there, mama’s cells "educate" developing infant cells to attack the same infectious organisms to which mom was exposed. A process called, “maternal educational immunity”.
Thymus aging
The thymus starts to shrink after puberty — a process that continues throughout lifetime. Aging-related inflammation leads to diminution of a critical structural protein called lamin-B1. It is required for thymic epithelial cells to develop and maintain normal function.
Zinc for T-cell development and thymic regeneration
But hope is on the horizon for repair and regeneration.

Prolonged lymphopenia represents a major clinical problem after cytoreductive therapies such as chemotherapy and the conditioning required for hematopoietic stem cell transplant (HCT), contributing to the risk of infections and malignant relapse. Restoration of T-cell immunity depends on tissue regeneration in the thymus, the primary site of T-cell development, although the capacity of the thymus to repair itself diminishes over its lifespan. However, although boosting thymic function and T-cell reconstitution is of considerable clinical importance, there are currently no approved therapies for treating lymphopenia. Here we found that zinc (Zn) is critically important for both normal T-cell development and repair after acute damage. Accumulated Zn in thymocytes during development was released into the extracellular milieu after HCT conditioning, where it triggered regeneration by stimulating endothelial cell production of BMP4 via the cell surface receptor GPR39. Dietary supplementation of Zn was sufficient to promote thymic function in a mouse model of allogeneic HCT, including enhancing the number of recent thymic emigrants in circulation although direct targeting of GPR39 with a small molecule agonist enhanced thymic function without the need for prior Zn accumulation in thymocytes. Together, these findings not only define an important pathway underlying tissue regeneration but also offer an innovative preclinical approach to treat lymphopenia in HCT recipients. - L Iovino et al.

Clinical trials using oral zinc supplements are underway for treatment pre- and post- transplant. Likely, it will also be of use for those of us with aged glands.
~~~~~~~~~~~~~~~~~~~~~~~~
Hmmmm… do these “thymus” flowers resemble the gland?
REFERENCES
KA Kooshesh et al. Health Consequences of Thymus Removal in Adults. New England Journal of Medicine (2023). DOI:10.1056/NEJMoa2302892
https://www.verywellhealth.com/thyroid-disease-after-thymus-removal-4159741
M Paolino et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy, Nature (2020). DOI: 10.1038/s41586-020-03071-0 , www.nature.com/articles/s41586-020-03071-0
MK Ghosh et al. Maternal Milk T Cells Drive Development of Transgenerational Th1 Immunity in Offspring Thymus, The Journal of Immunology (2016). DOI: 10.4049/jimmunol.1502483
S Yue et al. Cell‐type‐specific role of lamin‐B1 in thymus development and its inflammation‐driven reduction in thymus aging, Aging Cell (2019). DOI: 10.1111/acel.12952
L Iovino et al. Activation of the Zinc-sensing receptor GPR39 promotes T cell reconstitution after hematopoietic cell transplant in mice. Blood (2022). DOI: 10.1182/blood.2021013950. ashpublications.org/blood/arti 182/blood.2021013950
E Perkey, I Maillard. Zinc: a damage signal promoting thymic repair. Blood (2022) 139 (25): 3569–3570. https://doi.org/10.1182/blood.2022016333






Thank you for expanding my knowledge base. The Th1 aspect pricked my curiosity. I use thyme tea for my asthma (very tasty by the by). The flower does resemble the thymus the same way the hawthorn flower resembles the heart. Plants are fascinating that way. The more I know the less I have to rely on my "healthcare team".