Migraine Headache - focus on the trigeminal nerve
Triggers initiate a burst of slow wave propagation across its network.
Migraine is a complex neurological disease that is characterized by attacks lasting between 4 hours and 3 days, and is characterized primarily by a moderate‐to‐severe paroxysmal, unilateral headache that is aggravated by movement, and may be accompanied by symptoms of photophobia, phonophobia, osmophobia, allodynia, pain on movement, and nausea and vomiting. A person with migraine has a hypersensitive nervous system. It overreacts to stimuli, causing a wave of brain activity that leads to a headache and other symptoms. The trigeminal nerve is involved in attacks for almost all people with migraine. It is a network of wiring that attaches to special sensors located in our:
Facial Skin
Mucous Membranes
Muscles
Tendons
Teeth
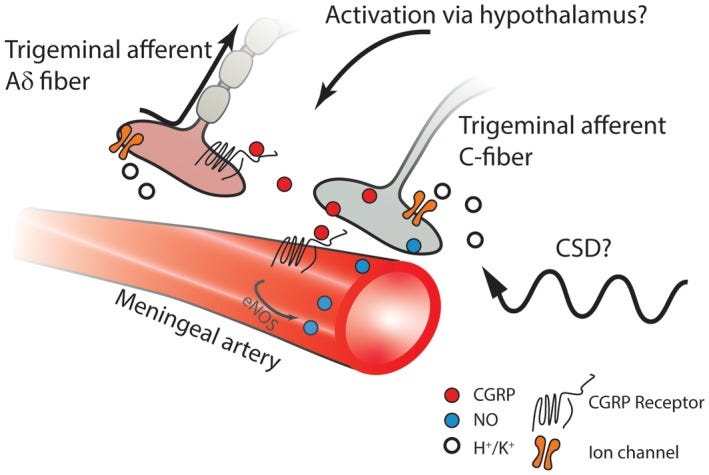
The meningeal vasculature is densely innervated with primary afferent nociceptive unmyelinated C‐fibers and thinly myelinated Aδ fibers arising predominantly from the ophthalmic (V1) division of the trigeminal nerve. These sensory nerves have cell bodies in the trigeminal ganglion (TG) and bifurcating axons that project to peripheral and central sites. Their peripheral fibers innervate the cerebral arteries, meningeal arteries, and project into parts of the dura that are not highly vascularized.
Originally, migraine headache was classified as a vascular disorder, resulting from meningeal vasodilation. Now it is seen as a complex neurological disease that includes the participation of multiple cortical, subcortical, and brainstem areas that regulate autonomic, affective, cognitive, and sensory functions.
Aura Spreading Depression
Migraine with aura is a common and debilitating neurological disorder, consisting of severe head pain and sensory amplifications. For a third of migraineurs, the headache is preceded by an aura, the earliest physiologically measurable feature of the migraine attack. It is a spreading sensory hallucination, caused by a spreading depolarization (SD) of brain tissue [cortical spreading depression]. Since it can initiate headache mechanisms in experimental animal models, it can be induced and then measured.

Recent research in a genetic mouse model of migraine shows plumes of neurotransmitter glutamate release into the extra-cellular space, arising from inefficient glutamate clearance by astrocytes. The study found that plumes precede the onset of spreading depolarization. They propose a model in which the depolarizing stimulus releases enough glutamate to overwhelm the binding capacity of astrocytic glutamate transporters, resulting in cooperative activation of synaptic and extrasynaptic NMDA receptors in sufficient number to initiate the positive feedback cycle that ignites SD.
Glutamate release during plumes is likely due to Ca2+-mediated vesicular release from neurons. Stimuli that depolarize neural membranes (KCl and SD) are sufficient to induce plumes. As SD propagates into the surrounding cortex, plumes occur during the depolarization phase, behind the leading wavefront.
Trigeminal Involvement
The trigeminal ganglion neurons provide the connection between the periphery, stemming from the interface between the primary afferent fibers of the trigeminal ganglion and the meningeal vasculature and the central terminals in the trigeminal nucleus caudalis. The neuropeptide CGRP is abundant in trigeminal ganglion neurons, and is released from the peripheral nerve and central nerve terminals as well as being secreted within the trigeminal ganglion. Release of CGRP from the peripheral terminals initiates a cascade of events that include increased synthesis of nitric oxide and sensitization of the trigeminal nerves. Secreted CGRP in the trigeminal ganglion interacts with adjacent neurons and satellite glial cells to perpetuate peripheral sensitization, and can drive central sensitization of the second-order neurons. A shift in central sensitization from activity-dependent to activity-independent central sensitization may indicate a mechanism driving the progression of episodic migraine to chronic migraine. The pathophysiology of cluster headache is much more obscure than that of migraine, but emerging evidence suggests that it may also involve hypersensitivity of the trigeminovascular system. -S Iyengar et al
Allergy Triggers Migraine
A recent study highlights the direct relationship of rhinitis—irritation and inflammation of the nasal mucus membrane caused by allergic and non- allergic triggers—to the frequency of migraine headaches attacks. Asthmatic patients, too, are more likely to also have allergies and prior studies pointed out that patients with allergies might be prone to more frequent headaches. Patients with asthma may have an overactive parasympathetic nervous system that predisposes them to attacks of both migraine and asthma.
Bummer … pollen does comes with the flowers of spring. So, it is important to nip those allergy symptoms in the bud. Past newsletters did discuss the management of mast cells and gives advice on how to muster up resources to get through, despite them.
Those blooming ends will justify the means.
biomedworks.substack.com/p/hydrogel-in-alveoli-covid-pneumonia
biomedworks.substack.com/p/blood-clots-and-sars-cov-2-mast-cells
REFERENCES
PD Parker et al. Non-canonical glutamate signaling in a genetic model of migraine with aura. Neuron Vol 109, ISSUE 4, P611-628.e8, February 17, 2021 https://www.cell.com/neuron/fulltext/S0896-6273(20)30896-5
S Iyengar et al. CGRP and the Trigeminal System in Migraine Headache. 2019 May; 59(5): 659–681. doi: 10.1111/head.13529. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6593989/
Got the sniffles? Migraines spike with allergies and hay fever (2013, November 25) https://medicalxpress.com/news/2013-11-sniffles-migraines-spike-allergies-hay.html
Beware, asthma sufferers: Migraines may worsen (2015, November 30) https://medicalxpress.com/news/2015-11-beware-asthma-migraines-worsen.html





