Renal Regeneration via salt and fluid manipulations
The macula densa sends signals for regrowth
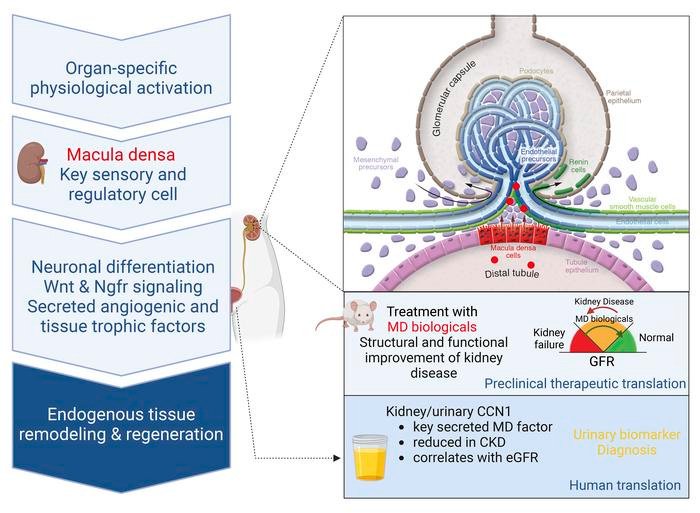
Two recent articles report that physiological fluxuations of salt and water can stimulate new growth in the kidney. The sensor cells of the glomerulus, macula densa (MD), are the signal generators for this phenomenon, using regeneration and repair pathways to reverse kidney injury.
Tissue regeneration is limited in several organs, including the kidney, contributing to the high prevalence of kidney disease globally. However, evolutionary and physiological adaptive responses and the presence of renal progenitor cells suggest an existing remodeling capacity. This study uncovered endogenous tissue remodeling mechanisms in the kidney that were activated by the loss of body fluid and salt and regulated by a unique niche of a minority renal cell type called the macula densa (MD). Here, we identified neuronal differentiation features of MD cells that sense the local and systemic environment and secrete angiogenic, growth, and extracellular matrix remodeling factors, cytokines and chemokines, and control resident progenitor cells. Serial intravital imaging, MD nerve growth factor receptor and Wnt mouse models, and transcriptome analysis revealed cellular and molecular mechanisms of these MD functions. Human and therapeutic translation studies illustrated the clinical potential of MD factors, including CCN1, as a urinary biomarker and therapeutic target in chronic kidney disease. The concept that a neuronally differentiated key sensory and regulatory cell type responding to organ-specific physiological inputs controls local progenitors to remodel or repair tissues may be applicable to other organs and diverse tissue-regenerative therapeutic strategies. - G Gyarmati, et al.
In their mice models, low-salt diet induced formation of axon-like cell projections from the basal surface of MD cells. Low sodium in tubular fluid triggered MD cells to release angiogenic and growth factors, such as CCN1.

The macula densa (MD) is a distinct cluster of approximately 20 specialized kidney epithelial cells that constitute a key component of the juxtaglomerular apparatus. Unlike other renal tubular epithelial cell populations with functions relating to reclamation or secretion of electrolytes and solutes, the MD acts as a cell sensor, exerting homeostatic actions in response to sodium and chloride changes within the tubular fluid. Electrolyte flux through apical sodium transporters in MD cells triggers release of paracrine mediators, affecting blood pressure and glomerular hemodynamics. In this issue of the JCI, Gyarmati and authors explored a program of MD that resulted in activation of regeneration pathways. Notably, regeneration was triggered by feeding mice a low-salt diet. Furthermore, the MD cells showed neuron-like properties that may contribute to their regulation of glomerular structure and function. These findings suggest that dietary sodium restriction and/or targeting MD signaling might attenuate glomerular injury. - Y Xia, TM Coffman.
Using a mouse model of adriamycin-associated glomerulopathy [focal glomerulosclerosis], exogenous infusions of CCN1 attenuated the severity of glomerular pathology, as seen with reduced albuminuria. They also treated these mice with MD cells grown in low-salt conditions. The MD cell treatment producing the bigger improvements in kidney structure and function, which could be due to the MD cells secreting not only CCN1, but also additional unknown factors that promote kidney regeneration.
This investigation of MD cell–mediated tissue regeneration was dependent on Wnt/β-catenin signaling, which is a key regulator of kidney embryogenesis. There seems to be interaction between MD cells and the local sympathetic nervous system, which may be involved in the growth. Authors proposed that sympathetic innervation may modulate regenerative responses in other tissues [e.g. skin would healing], as well.
Other BioMedWorks newsletters with information about kidneys:
Key kidney care leading to longevity. Starts with good eats.
Wonders of Intermittent Fasting. Short or long - it all works.
REFERENCES
G Gyarmati, et al. Neuronally differentiated macula densa cells regulate tissue remodeling and regeneration in the kidney. Journal of Clinical Investigation (2024). DOI: 10.1172/JCI174558
Y Xia, TM Coffman. Hold the salt for kidney regeneration. J Clin Invest. 2024;134(11):e181397. https://doi.org/10.1172/JCI181397.




And here I was taught that the only regenerative organ was the liver. Thanks for information. Appreciated!
Hi, BioMedWorks!
How would one implement this insight and information? I got acute glomerulo-nephritis back in the nursing school 50 years ago from strep when no antibiotic would work. Allergic to them all. 52 R and 54% L damage at that time. I've been blessed these last many years with no problems at all, but now find myself in stage 3-4 kidney disease. Any tips or thoughts? If you have the time, you can reach me at jacque@deeprootsathome.com I would kindly appreciate anything you have. Blessings,
Jacqueline